1. Biography
Amedeo Avogadro's life spanned a period of significant political and scientific change in Italy, marked by his dedication to both law and natural sciences, as well as his involvement in the political landscape of his time.

1.1. Birth and Early Life
Amedeo Avogadro was born in Turin, then part of the Kingdom of Sardinia (now Italy), on August 9, 1776. He came from a noble family; his father, Filippo Avogadro, was a legal scholar and a prominent figure in the Sardinian government, while his mother was Anna Vercellone.
1.2. Education
Avogadro initially pursued a career in law, graduating with a degree in ecclesiastical law at the age of 20 or 21 in 1796. After practicing law for several years, he developed a profound interest in physics and mathematics, which were then often referred to as 'positive philosophy'. He embarked on a path of self-study in these scientific disciplines around 1800.
1.3. Early Career and Academic Pursuits
Following his legal practice, Avogadro began his academic career. In 1803, he submitted his first scientific paper on electrical engineering to the Turin Academy of Sciences. His dedication to science led to his appointment as an assistant professor at the University of Turin in 1806. By 1809, he became a professor of natural philosophy at the Royal Vercelli College in Vercelli, where his family owned property. It was during this period, in 1811, that he published his seminal article, Essai d'une manière de déterminer les masses relatives des molécules élémentaires des corps, et les proportions selon lesquelles elles entrent dans ces combinaisons ("Essay on a manner of Determining the Relative Masses of the Elementary Molecules of Bodies and the Proportions by Which They Enter These Combinations"), which contained what would later be known as Avogadro's hypothesis. He submitted this essay to Jean-Claude Delamétherie's French publication, Journal de Physique, de Chimie et d'Histoire naturelle ("Journal of Physics, Chemistry and Natural History").
1.4. Professorial Career and Political Involvement
In 1820, Avogadro was appointed the first professor of mathematical physics at the University of Turin. Turin had been re-established as the capital of the restored Savoyard Kingdom of Sardinia under Victor Emmanuel I after the fall of Napoleon in 1815. Avogadro became actively involved in the revolutionary movement of March 1821, which sought to establish a constitutional monarchy. As a direct consequence of his political activities, he lost his professorial chair in 1823. The university officially stated that it was "very glad to allow this interesting scientist to take a rest from heavy teaching duties, in order to be able to give better attention to his researches," a diplomatic phrasing for his dismissal. However, his academic career was later restored. In 1833, he was recalled to the University of Turin, where he continued to teach for another two decades, until his retirement in 1850. Eventually, King Charles Albert granted a Constitution, known as the Statuto Albertino, in 1848.
1.5. Public Service and Scientific Administration
Beyond his academic and scientific pursuits, Avogadro also engaged in public service. He held various administrative positions, contributing to areas such as statistics, meteorology, and weights and measures. Notably, he was instrumental in introducing the metric system to Piedmont, demonstrating his commitment to standardization and societal infrastructure. He also served as a member of the Royal Superior Council on Public Instruction.
1.6. Personal Life
Little is known about Avogadro's private life, which is generally described as sober and religious. He married Felicita Mazzé and together they had six children. Some historical accounts suggest he may have sponsored Sardinian revolutionaries, a movement that eventually saw the announcement of Charles Albert's constitution.
1.7. Death
Amedeo Avogadro died in Turin on July 9, 1856, at the age of 79.
2. Scientific Achievements
Avogadro's scientific contributions were fundamental to the development of modern chemistry and physics, primarily through his molecular theory.
2.1. Avogadro's Law
Avogadro's most renowned contribution is the principle known as Avogadro's law. This law states that under the same conditions of temperature and pressure, equal volumes of all gases contain the same number of molecules. He developed this hypothesis in 1811, building upon Joseph Louis Gay-Lussac's law on combining volumes of gases, published in 1808.
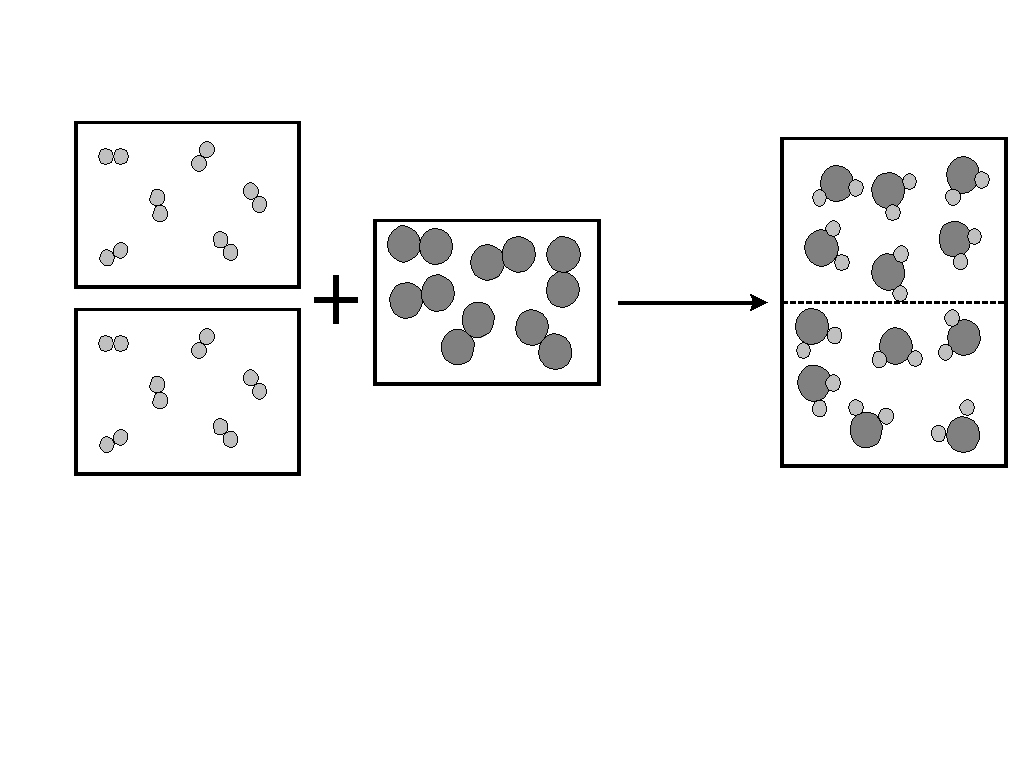
At the time, a major challenge in chemistry was the confusion surrounding the concepts of atoms and molecules. John Dalton's atomic theory, while groundbreaking, struggled to explain certain gas reactions, such as the observation that two volumes of hydrogen react with one volume of oxygen to produce two volumes of water vapor (at the same temperature and pressure). According to Dalton's initial assumption that elemental particles were single atoms, this reaction would be represented as H + O → HO, which did not align with the experimental volume ratios of 2:1:2.
Avogadro's law resolved this by proposing two key ideas:
- First, that equal volumes of gases contain equal numbers of particles.
- Second, and crucially, that gaseous elements like hydrogen and oxygen exist not as single atoms but as diatomic molecules (e.g., H₂ and O₂).
With these insights, the reaction could be correctly represented as 2H₂ + O₂ → 2H₂O, perfectly matching the observed volume ratios. This allowed for the correct determination of molecular formulas for various compounds.
2.2. Distinction Between Atoms and Molecules
One of Avogadro's most significant conceptual contributions was his clear differentiation between atoms and molecules. Before his work, the terms "atom" and "molecule" were often used interchangeably, leading to ambiguity in early atomic theory. Avogadro clarified that gases are composed of molecules, and these molecules, in turn, are composed of atoms. He introduced the concept of an "elementary molecule" to refer to what we now call an atom. This distinction was critical for understanding chemical reactions and the composition of matter, as it allowed for the correct interpretation of combining volumes and masses. He also paid significant attention to the definition of mass, distinguishing it from weight.
2.3. Avogadro Constant
In honor of Avogadro's contributions to molecular theory, the number of elementary entities (atoms, molecules, ions, or other particles) in one mole of a substance is named the Avogadro constant, denoted as NA. Its exact value is 6.02214076 × 1023. The Avogadro constant is fundamental in chemistry and physics, enabling chemists to accurately calculate the amounts of substances involved or produced in chemical reactions. While named after Avogadro, the first calculation of this constant's value was performed by Johann Josef Loschmidt, sometimes referred to as the Loschmidt number in German-speaking countries.
2.4. Major Publications
Avogadro's scientific output included several key publications that detailed his theories and experimental findings:
- His seminal 1811 essay, Essai d'une manière de déterminer les masses relatives des molécules élémentaires des corps, et les proportions selon lesquelles elles entrent dans ces combinaisons, laid out his hypothesis on molecular volumes.
- In 1815, he published Mémoire sur les masses relatives des molécules des corps simples, ou densités présumées de leur gaz, et sur la constitution de quelques-uns de leur composés, pour servir de suite à l'Essai sur le même sujet, publié dans le Journal de Physique, juillet 1811 ("Note on the Relative Masses of Elementary Molecules, or Suggested Densities of Their Gases, and on the Constituents of Some of Their Compounds, As a Follow-up to the Essay on the Same Subject, Published in the Journal of Physics, July 1811"), focusing on gas densities.
- He followed this with Nouvelles considérations sur la théorie des proportions déterminées dans les combinaisons, et sur la détermination des masses des molécules des corps ("New Considerations on the Theory of Proportions Determined in Combinations, and on Determination of the Masses of Atoms") in 1821.
- Shortly thereafter, he published Mémoire sur la manière de ramener les composès organiques aux lois ordinaires des proportions déterminées ("Note on the Manner of Finding the Organic Composition by the Ordinary Laws of Determined Proportions").
- His comprehensive work, Fisica dei corpi ponderabili, ossia Trattato della costituzione materiale de' corpi (Physics of Ponderable Bodies, or Treatise on the Material Constitution of Bodies), was published in four volumes between 1837 and 1841.
2.5. Other Scientific Investigations
Beyond his groundbreaking work in molecular theory, Avogadro conducted research in a variety of other scientific fields. His interests included electricity, the distillation of liquids, specific heat, capillary action, and the volume of atoms. He also applied his laws to metals, calculating the atomic weights of 17 different types of metals.
3. Reception and Re-evaluation of Theory
Despite its profound implications, Avogadro's molecular theory initially faced widespread indifference and obscurity within the scientific community. It was not immediately accepted, and its significance remained largely unrecognized for decades after its initial publication.
3.1. Early Indifference and Obscurity
Upon its publication in 1811, Avogadro's hypothesis received little attention from the scientific establishment. This neglect was partly due to the complex and perhaps unfamiliar language of his papers, influenced by his background in law, which made his arguments difficult for contemporary chemists to grasp. Additionally, some related experiments with inorganic substances seemed to contradict his law, further hindering its acceptance. Even André-Marie Ampère proposed a very similar theory independently in 1813, but it too was met with the same indifference. For much of his life, Avogadro remained relatively unknown outside of his immediate academic circles.
3.2. Later Recognition and Vindication
The vindication of Avogadro's work came posthumously, largely through the efforts of other prominent scientists. The pivotal moment arrived at the Karlsruhe Congress in 1860, four years after Avogadro's death. At this congress, Italian chemist Stanislao Cannizzaro presented a paper, "Sketch of a Course of Chemical Philosophy," which effectively clarified and championed Avogadro's hypothesis. Cannizzaro explained that the apparent contradictions observed with inorganic substances were due to molecular dissociations at certain temperatures, and he demonstrated how Avogadro's law could be used to determine not only molecular masses but also atomic masses.
Studies by Charles Frédéric Gerhardt and Auguste Laurent in organic chemistry also played a crucial role, demonstrating how Avogadro's law explained why equal quantities of molecules in a gas occupy the same volume. Further evidence supporting Avogadro's law was provided by Rudolf Clausius with his kinetic theory of gases, proposed in 1857. Additionally, Jacobus Henricus van 't Hoff showed that Avogadro's theory also held true in dilute solutions.
The widespread recognition of Avogadro's immense contribution to chemistry finally came in 1911, when a meeting in Turin commemorated the hundredth anniversary of his classic 1811 paper. The event was attended by King Victor Emmanuel III, formally acknowledging Avogadro's profound impact on the field.
4. Impact and Legacy
Avogadro's scientific contributions have had a lasting and profound influence on the development of chemistry and physics, establishing him as a foundational figure in modern science.
4.1. Foundation of Modern Chemistry
Avogadro is widely hailed as a founder of the atomic-molecular theory. His law and the conceptual distinction between atoms and molecules provided the essential groundwork for understanding the composition of matter and how chemical reactions occur. By clarifying these fundamental concepts, Avogadro's work enabled the accurate determination of atomic and molecular weights and paved the way for the quantitative analysis that underpins modern chemistry. His principles became indispensable for chemical advancements and continue to be a cornerstone of scientific education and research worldwide.
4.2. Commemoration and Honors
Avogadro's enduring legacy is honored in various ways within the scientific community. The most prominent tribute is the naming of the Avogadro constant (NA) after him, which is a fundamental constant in chemistry and physics. Beyond this, his name is commemorated in other scientific contexts, including the mineral Avogadrite and a lunar crater named Avogadro. These honors ensure that his memory and groundbreaking achievements continue to be recognized and celebrated.