1. Overview
Sir Humphry Davy, 1st Baronet (1778-1829) was a prominent British chemist and inventor whose pioneering work significantly advanced the fields of chemistry and industrial safety. He is widely recognized for his groundbreaking use of electrolysis to isolate several chemical elements for the first time, including potassium and sodium in 1807, followed by calcium, strontium, barium, magnesium, and boron in 1808 and 1809. Davy also definitively established chlorine and iodine as elements, challenging existing chemical theories. His practical inventions, most notably the Davy lamp, revolutionized safety in coal mines by preventing explosions caused by flammable gases.
Beyond his direct discoveries, Davy's research laid the foundation for the new field of electrochemistry. He also conducted extensive studies on the physiological effects of gases, identifying the anaesthetic properties of nitrous oxide, which he famously called "laughing gas." A highly influential lecturer at the Royal Institution in London, Davy captivated audiences with his dramatic demonstrations and eloquent explanations, contributing significantly to the public's understanding and appreciation of science. His career culminated in his presidency of the Royal Society, the highest scientific honor in Britain at the time. Despite controversies later in his life, including disputes over the Davy lamp's invention and a strained relationship with his protégé Michael Faraday, Davy's legacy as a scientific pioneer and a key figure in the development of modern chemistry remains profound.
2. Early Life and Education
Humphry Davy's early life in Cornwall, England, laid the foundation for his remarkable scientific career, marked by a keen intellect and a self-driven pursuit of knowledge.
2.1. Childhood and Education
Humphry Davy was born on December 17, 1778, in Penzance, Cornwall, England, as the eldest of five children to Robert Davy, a woodcarver, and Grace Millett. His hometown of Penzance was characterized by a strong belief in the supernatural and a limited appreciation for literature and science among the middle and upper classes, who often preferred activities like hunting and cockfighting.
At the age of six, Davy began attending grammar school in Penzance. Three years later, his family relocated to Varfell, near Ludgvan, where Davy boarded with his godfather and future guardian, John Tonkin, during term-time. After leaving grammar school in 1793, Tonkin funded his attendance at Truro Grammar School under the guidance of Reverend Dr. Cardew. Cardew, however, later dryly remarked that he "could not discern the faculties by which he was afterwards so much distinguished." Davy found formal learning restrictive, stating in a letter to his mother, "Learning naturally is a true pleasure; how unfortunate then it is that in most schools it is made a pain." He considered it fortunate that he was "left much to myself as a child, and put upon no particular plan of study... What I am I made myself." His brother, John Davy, a chemist himself, noted Humphry's "native vigour" and "the genuine quality of genius." During his school days, Davy entertained friends by writing poetry, composing Valentines, and recounting stories from One Thousand and One Nights.
2.2. Apprenticeship and Early Scientific Inquiries
Following his father's death in 1794, John Tonkin apprenticed the 16-year-old Davy to John Bingham Borlase, a surgeon with a practice in Penzance. While working in the apothecary's dispensary, Davy began conducting his initial chemical experiments at home. These early endeavors often annoyed his family and friends; his older sister complained about corrosive substances damaging her dresses, and a friend humorously predicted that the "incorrigible" Davy would eventually "blow us all into the air."
In 1797, after learning French from a refugee priest, Davy read Lavoisier's Traité élémentaire de chimie, a work that profoundly influenced his future research, much of which can be seen as a reaction against Lavoisier's theories and the dominance of French chemists.
Davy also pursued his passion for poetry, writing over 160 manuscript poems, though most remained unpublished. These poems reflected his perspectives on his career and human life, touching on themes such as death, metaphysics, geology, natural theology, and chemistry. John Ayrton Paris, one of Davy's biographers, noted that his early poems "bear the stamp of lofty genius." His first preserved poem, "The Sons of Genius," dates to 1795, while later descriptive verses include "On the Mount's Bay" and "St Michael's Mount." As his health declined in later life, his poetry increasingly focused on immortality and death.
Around 1796, Davy also explored painting, with three of his works from this period now housed at the Penlee House museum in Penzance. These include a view from above Gulval showing the church, Mount's Bay, and St Michael's Mount, as well as two depictions of Loch Lomond in Scotland.
At 17, Davy engaged in discussions about the materiality of heat with his Quaker friend and mentor, Robert Dunkin. Dunkin once remarked, "I tell thee what, Humphry, thou art the most quibbling hand at a dispute I ever met with in my life." Dunkin demonstrated the concept of heat generated by motion by rubbing two plates of ice together by the Lariggan River in Penzance, showing how they melted and then re-united by regelation when the motion ceased. Davy later refined and replicated many of these ingenious experiments, learned from Dunkin, in his lectures at the Royal Institution.
3. Early Career and Scientific Development
Davy's entry into the scientific community marked a period of rapid development, characterized by his innovative research at key institutions and his burgeoning public reputation.
3.1. Work at the Pneumatic Institution
Davy's scientific talents began to gain recognition through his connections in Cornwall. Davies Giddy (later Davies Gilbert), a Fellow of the Royal Society, encountered Davy in Penzance and, impressed by his conversation, invited him to use his library. This led to Davy's introduction to Dr. Edwards, a chemistry lecturer at St. Bartholomew's Hospital's school, who allowed Davy to use his laboratory. Dr. Edwards also drew Davy's attention to the rapid decay of copper and iron floodgates at Hayle port due to seawater, a phenomenon of galvanic corrosion not yet understood, which prepared Davy for later experiments on ships' copper sheathing. Gregory Watt, son of James Watt, also befriended Davy during a stay in Penzance and provided him with chemistry instruction. Davy was also acquainted with the Wedgwood family.
Physician and scientific writer Thomas Beddoes and geologist John Hailstone, engaged in a geological controversy, met Davy during a survey of the Cornish coast with Giddy. Beddoes, who had established the 'Pneumatic Institution' in Bristol for medical research on "factitious airs" (artificially produced gases), was seeking an assistant to oversee the laboratory. Giddy recommended Davy, and in 1798, Gregory Watt presented Beddoes with Davy's Young man's Researches on Heat and Light. After negotiations, Davy's mother and Borlase consented to his departure, despite Tonkin's desire for Davy to remain a surgeon in Penzance.
On October 2, 1798, Davy joined the Pneumatic Institution. The generous terms of his arrangement allowed him to relinquish his claims on his paternal property in favor of his mother. Although he initially intended to pursue a medical degree at Edinburgh, he soon began to fill parts of the institution with voltaic batteries. During his time in Bristol, Davy also formed a long romantic friendship with Anna Beddoes, Thomas Beddoes' wife and novelist Maria Edgeworth's sister, who guided him on local excursions and exchanged poems with him.
In 1799, Davy's essay On Heat, Light, and the Combinations of Light, along with On Phos-oxygen and its Combinations and Theory of Respiration, were published in the first volume of West-Country Collections. On February 22, 1799, Davy wrote to Davies Giddy, expressing his conviction in "the non-existence of caloric as I am of the existence of light."
Davy gained increasing recognition for his experiments on the physiological effects of gases, particularly nitrous oxide. This gas, first synthesized by Joseph Priestley in 1772, was called "dephlogisticated nitrous air." On April 10, 1799, Davy informed Giddy of his discovery of a method for purifying nitrous oxide, which he found "perfectly respirable when pure" and capable of causing intoxication. He recounted breathing 16 quarts of it for nearly seven minutes, stating it "absolutely intoxicated me." His enthusiastic experimental subjects included his poet friends Robert Southey and Samuel Taylor Coleridge, as well as Gregory Watt and James Watt, who even built a portable gas chamber for Davy's experiments. Davy noted that nitrous oxide might be useful for surgical operations, commenting in his 1800 Researches, Chemical and Philosophical that it "appears capable of destroying pain." However, anesthesia was not regularly adopted in medicine or dentistry until decades after Davy's death.
Davy's gas experiments were not without risk. His inhalation of nitric oxide severely injured his mucous membrane, and an attempt to inhale four quarts of "pure hydrocarbonate" (carbon monoxide) left him "sinking into annihilation," barely able to articulate "I do not think I shall die" upon removal to open air. He recovered after several hours, noting his pulse as "threadlike and beating with excessive quickness."
In 1799, Beddoes and Davy published Contributions to physical and medical knowledge and Essays on heat, light, and the combinations of light. These early works were harshly criticized for their poor experimental basis, leading Davy to regret publishing what he later called "the dreams of misemployed genius." These criticisms, however, spurred Davy to refine his experimental techniques, dedicating more time to rigorous experimentation. In December 1799, Davy made his first visit to London, expanding his circle of acquaintances, including William Godwin. By 1800, Davy was successfully repeating galvanic experiments and published his more positively received Researches, Chemical and Philosophical, chiefly concerning Nitrous Oxide and its Respiration. He also assisted William Wordsworth and Samuel Taylor Coleridge by proofreading the second edition of their Lyrical Ballads in 1800, though the edition still contained errors.
3.2. Work at the Royal Institution
In 1799, Benjamin Thompson (Count Rumford) proposed establishing the Royal Institution in London, an 'Institution for Diffusing Knowledge' aimed at promoting scientific understanding among the public. The building on Albemarle Street was acquired in April 1799, with Thompson serving as secretary and Dr. Thomas Garnett as the first lecturer.
In February 1801, Davy was interviewed by the Royal Institution's committee, including Joseph Banks, Benjamin Thompson, and Henry Cavendish. On March 8, 1801, Davy wrote to Davies Giddy about offers from Banks and Thompson, discussing a potential move to London and funding for his work in galvanism, and indicating a likely end to his collaboration with Beddoes on therapeutic gases. The following day, Davy left Bristol to assume his new role at the Royal Institution as assistant lecturer in chemistry, director of the chemical laboratory, and assistant editor of the institution's journals, with a salary of 100 GBP per annum, along with accommodation and provisions.
On April 25, 1801, Davy delivered his first lecture on 'Galvanism'. Influenced by his conversations with Coleridge on the nature of knowledge and progress, Davy presented a vision of human civilization advanced by scientific discovery, emphasizing science as a "creative" power that enables humanity to "interrogate nature with power, not simply as a scholar... but rather as a master, active with his own instruments." His initial lecture received rave reviews, and by June, attendance reached nearly 500 people, with Davy exclaiming, "There was Respiration, Nitrous Oxide, and unbounded Applause. Amen!" He relished his public status.

Davy's lectures were known for their spectacular and sometimes dangerous chemical demonstrations, delivered with considerable showmanship by the young and charismatic lecturer. He incorporated poetic and religious commentary, suggesting that God's design was revealed through chemical investigations, partly to appeal to the women in his audiences. Davy, like many contemporaries, supported female education and involvement in science, even proposing their admission to Royal Society evening events. His popularity among women in London was notable, as evidenced by a satirical cartoon by James Gillray showing nearly half the attendees at his lectures as female. This support, however, led to gossip and criticism, with some perceiving him as "unmanly."
In 1802, utilizing the most powerful electrical battery of its time at the Royal Institution, Davy created the first incandescent light by passing an electric current through a thin platinum strip, chosen for its high melting point. While not bright or long-lasting enough for practical use, it demonstrated the principle. By 1806, he showcased a more powerful form of electric lighting to the Royal Society in London: an early arc lamp that produced illumination from an electric arc between two charcoal rods.
Following his successful lecture series on Galvanism, Davy commenced a new series on Agricultural Chemistry, further boosting his popularity. By June 1802, after just over a year at the Institution and at the age of 23, Davy was promoted to full lecturer, and Dr. Garnett quietly resigned due to health reasons.
In November 1804, Davy became a Fellow of the Royal Society, an institution he would later preside over. He was also a founding member of the Geological Society of London in 1807 and became a Fellow. His international recognition grew with his election as a foreign member of the Royal Swedish Academy of Sciences and an honorary member of the American Philosophical Society in 1810, and a Foreign Honorary Member of the American Academy of Arts and Sciences in 1822.
4. Major Scientific Discoveries and Contributions
Davy's scientific career was marked by a series of revolutionary discoveries and practical inventions that fundamentally changed chemistry and improved industrial safety.
4.1. Electrochemistry and Element Discovery
Davy was a pioneer in the field of electrolysis, utilizing the newly invented voltaic pile to decompose common compounds and isolate numerous new elements. His work in this area was particularly groundbreaking, leading to the discovery of highly reactive metals.
4.1.1. Potassium and Sodium
In 1807, Davy achieved a major breakthrough by isolating potassium from caustic potash (potassium hydroxide, KOH) through electrolysis. Potassium was the first metal to be isolated by this method. Before the 19th century, potassium and sodium were not clearly distinguished. In the same year, Davy also successfully isolated sodium by passing an electric current through molten sodium hydroxide. These discoveries were significant advancements in understanding the alkali metals.


4.1.2. Barium, Calcium, Strontium, Magnesium, and Boron
Building on his success with alkali metals, Davy conducted further electrolysis experiments on alkaline earth metals during the first half of 1808, including lime (calcium oxide), magnesia (magnesium oxide), strontites (strontium oxide), and barytes (barium oxide). In early June, he received a letter from the Swedish chemist Berzelius, who, along with Dr. Pontin, had successfully obtained amalgams of calcium and barium by electrolyzing lime and barytes using a mercury cathode. Davy quickly replicated and expanded this method to strontites and magnesia. He observed that while these amalgams oxidized within minutes when exposed to air, they could be preserved for extended periods when submerged in naphtha, forming a white crust.
On June 30, 1808, Davy reported to the Royal Society that he had successfully isolated four new metals, which he named barium, calcium, strontium, and magnium (later changed to magnesium). These findings were subsequently published in the Philosophical Transactions. Although Davy conceded "magnium" was an "undoubtedly objectionable" name, he argued that "magnesium" was already applied to metallic manganese and wished to avoid ambiguity. The observations from these experiments also led to Davy isolating boron in 1809. Berzelius lauded Davy's 1806 Bakerian Lecture, "On Some Chemical Agencies of Electricity," as "one of the best memoirs which has ever enriched the theory of chemistry."
4.2. Discovery of Chlorine and Iodine
Davy's work extended to clarifying the elemental nature of substances previously misunderstood, challenging established chemical theories.
4.2.1. Chlorine

Chlorine was initially discovered in 1774 by Swedish chemist Carl Wilhelm Scheele, who called it "dephlogisticated marine acid" and mistakenly believed it contained oxygen. Scheele produced chlorine by reacting manganese dioxide (MnO2) with hydrochloric acid (HCl), observing its yellow-green color, distinct odor, and ability to decolorize litmus and kill insects, though he did not publish his findings. Davy, however, demonstrated that Scheele's substance, then known as oxymuriatic acid, contained no oxygen. This discovery directly contradicted Lavoisier's definition of acids as compounds containing oxygen. In 1810, Davy formally named the substance "chlorine," a name derived from the Greek χλωροςGreek, Ancient (chlōros), meaning "green-yellow," reflecting its characteristic color. He insisted that chlorine was, in fact, a chemical element.
4.2.2. Iodine
During his European tour in 1813, Davy investigated a mysterious substance isolated by Bernard Courtois in France. Attending lectures by Joseph Louis Gay-Lussac at the École Polytechnique on this substance, Davy quickly recognized its elemental nature. He subsequently wrote a paper for the Royal Society on the element, now known as iodine. This work, however, led to a dispute between Davy and Gay-Lussac over the priority of the research.
4.3. Research on Gases
Davy's early career at the Pneumatic Institution was heavily focused on the study of gases, particularly their physiological effects. He became increasingly well known for his experiments with nitrous oxide, which he nicknamed "laughing gas" due to its intoxicating effects. He was among the first to note its potential as an anesthetic to relieve pain during surgery, although its widespread medical use did not occur until decades after his death.
He also conducted dangerous experiments with other gases, including carbon monoxide. His attempt to inhale four quarts of "pure hydrocarbonate" gas resulted in severe symptoms, making him feel as if he was "sinking into annihilation." Despite the risks, Davy meticulously documented his physiological responses, such as his "threadlike and excessively quick" pulse during the carbon monoxide incident.
4.4. Inventions
Beyond his fundamental chemical discoveries, Davy applied his scientific knowledge to develop practical inventions that had significant societal impact.
4.4.1. The Davy Lamp
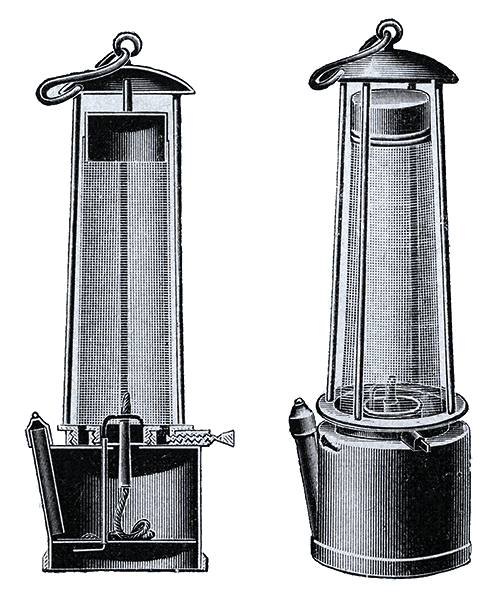

Upon his return to England in 1815, Davy turned his attention to developing safer lamps for coal mines. The Reverend Dr. Robert Gray of Bishopwearmouth in Sunderland, founder of the Society for Preventing Accidents in Coalmines, had urged Davy to use his chemical expertise to address the frequent mining explosions caused by firedamp (methane mixed with oxygen), which were often ignited by the open flames of miners' lamps. Tragedies like the Felling mine disaster of 1812, which killed 92 men, highlighted the urgent need for improved underground lighting.
Davy conceived the idea of enclosing a lamp's flame within an iron gauze. This design prevented the methane burning inside the lamp from igniting the surrounding atmosphere because the wire gauze rapidly absorbed the heat of the flame, cooling the gas below its ignition temperature. Although similar ideas for safety lamps had been demonstrated by William Reid Clanny and George Stephenson, Davy's specific use of wire gauze became widely adopted in subsequent lamp designs. Stephenson's lamp, popular in the north-east coalfields, also prevented flame propagation but through different means. Unfortunately, while the gauze lamp offered initial protection, it provided much less light and quickly deteriorated in the wet conditions of many pits due to rusting gauze, which could render the lamp unsafe. Some historical accounts suggest that the adoption of the Davy lamp, by allowing access to previously unworkable, gassier seams, paradoxically led to an increase in overall mining fatalities, as miners ventured into more dangerous areas.
There was some debate regarding whether Davy had independently discovered the principles behind his lamp or if he had been influenced by the work of Smithson Tennant; however, it was generally concluded that their work was independent. Davy notably refused to patent the lamp, believing its benefits should be freely available to save lives. For this invention, he was awarded the Rumford Medal in 1816.
4.4.2. Arc Lamp and Incandescent Light
In 1802, at the Royal Institution, Humphry Davy utilized what was then the world's most powerful electrical battery to create the first incandescent light. He achieved this by passing an electric current through a thin strip of platinum, a metal chosen for its exceptionally high melting point. While this early incandescent light was neither bright enough nor long-lasting for practical use, it successfully demonstrated the underlying principle of electric illumination. By 1806, Davy further advanced electric lighting by demonstrating a more powerful form of arc lamp to the Royal Society in London, which produced light from an electric arc generated between two charcoal rods. This invention, while still rudimentary, marked a significant step towards modern electric lighting.
4.5. Other Scientific Contributions
Davy's scientific curiosity spanned various disciplines, leading to diverse contributions beyond his most famous discoveries.
In June 1802, Davy published "An Account of a Method of Copying Paintings upon Glass, and of Making Profiles, by the Agency of Light upon Nitrate of Silver. Invented by T. Wedgwood, Esq. With Observations by H. Davy" in the Journals of the Royal Institution of Great Britain. This paper described his and Wedgwood's experiments with the photosensitivity of silver nitrate. Davy noted that "images of small objects, produced by means of the solar microscope, may be copied without difficulty on prepared paper." While Josef Maria Eder credited Wedgwood as "the first photographer in the world" for applying silver nitrate to image making, he suggested that it was Davy who conceived the idea of photographic enlargement using a solar microscope to project images onto sensitized paper. Neither found a means of permanently fixing their images, and Davy did not pursue these early photographic discoveries further. The principle of image projection using solar illumination later formed the basis for the earliest photographic enlarger, the "solar camera."
In 1815, Davy proposed a new definition for acids, suggesting they were substances containing replaceable hydrogen ions-hydrogen that could be partly or totally replaced by reactive metals positioned above hydrogen in the reactivity series. According to his definition, when acids reacted with metals, they formed salts and hydrogen gas. Bases, conversely, were defined as substances that reacted with acids to form salts and water. These definitions proved highly effective and remained largely functional throughout most of the 19th century.
Davy also undertook experiments on fragments of the Herculaneum papyri before his departure for Naples in 1818. His initial trials showed promise; he reported to the Royal Society that placing a fragment of a brown manuscript in a chlorine atmosphere caused "immediate action," the papyrus smoked, turned yellow, and the letters became more distinct. Applying heat further separated the layers, releasing hydrochloric acid fumes. Encouraged by these early successes, Davy traveled to Naples to continue his research, accompanied by his wife. They visited Flanders, where Davy was invited by coal miners to speak, and Carniola (now Slovenia), which became his favorite Alpine retreat, before arriving in Italy. In Rome, they befriended Lord Byron before proceeding to Naples. His work initially resulted in "partially unrolling 23 MSS., from which fragments of writing were obtained." However, upon returning to Naples in December 1819 after a summer in the Alps, Davy found the Italian museum staff unhelpful and obstructive. He eventually abandoned further work on the papyri, citing that "the labour, in itself difficult and unpleasant, been made more so, by the conduct of the persons at the head of this department in the Museum."
During his European tour, while sojourning in Florence, Davy, with Michael Faraday's assistance, conducted a series of experiments using the Grand Duke of Tuscany's burning glass. He successfully used concentrated sunlight to ignite a diamond, definitively proving that it is composed of pure carbon.
Davy also contributed to naval technology. From 1761, copper plating had been fitted to the undersides of Royal Navy ships to protect the wood from shipworms. However, the copper gradually corroded in saltwater. Between 1823 and 1825, Davy, again assisted by Faraday, attempted to protect the copper through electrochemistry. He attached sacrificial pieces of zinc or iron to the copper, providing cathodic protection to the host metal. While this method successfully prevented corrosion, it was discovered that the protected copper became fouled quickly with marine growth, significantly impeding the ship's handling. The Navy Board adopted Davy's "protectors" in 1823, but by 1824, widespread fouling led the British Admiralty to order their removal by the end of 1825. Davy's scheme was thus seen as a public failure, as the absence of poisonous copper salts allowed barnacles and other marine creatures to attach to the hull, despite the success in preventing corrosion itself.
5. Career Advancement and Recognition
Davy's professional trajectory saw him rise to the pinnacle of the British scientific establishment, earning numerous accolades and leading the most prestigious scientific society of his time.
5.1. Presidency of the Royal Society
On October 20, 1818, Davy was created a baronet, the first such honor conferred on a man of science in Britain, signifying his elevated status. This was followed a year later by his election as President of the Royal Society on November 30, 1820. The Society was then undergoing a transition from a gentlemen's club interested in natural philosophy to an academy representing increasingly specialized sciences. The previous president, Joseph Banks, had held the post for over 40 years, presiding autocratically over what was termed the "Banksian Learned Empire," which heavily emphasized natural history. Banks had intended for Davies Gilbert to succeed him and maintain the status quo, but Gilbert declined. Other candidates, including Prince Leopold of Saxe-Coburg and the Whig Edward St Maur, 11th Duke of Somerset, also withdrew. Davy, despite some fellows disapproving of his popularizing work at the Royal Institution, was the most outstanding scientist available.
Davy's election was unopposed, but underlying factional difficulties persisted. The "Cambridge Network" of mathematicians, including Charles Babbage and John Herschel, had supported William Hyde Wollaston and sought to block Davy, fearing he would not sufficiently encourage aspiring young mathematicians, astronomers, and geologists who were forming specialist societies. At 41, Davy's youth also raised concerns about another long presidency.
In his early years as president, Davy was optimistic about reconciling reformers and Banksians, stating in his first speech, "I trust that, with these new societies, we shall always preserve the most amicable relations... I am sure there is no desire in [the Royal Society] to exert anything like patriarchal authority in relation to these institutions."

However, Davy's reputation declined following failures such as his research into copper-bottomed ships, which diminished his popularity and authority. This was compounded by several political missteps. In 1825, his promotion of the new Zoological Society, of which he was a founding fellow, alienated expert zoologists by courting the landed gentry. He further offended mathematicians and reformers by failing to ensure Babbage received one of the new Royal Medals (a project he initiated) or the vacant secretaryship of the Society in 1826. In 1826, Davy suffered a stroke, from which he never fully recovered. By November 1826, the mathematician Edward Ryan noted widespread discontent, with "The Society, every member almost... in the greatest rage at the President's proceedings and nothing is now talked of but removing him." Despite this, he was re-elected unopposed but was visibly unwell. In January 1827, he traveled to Italy for his health, which did not improve. As the 1827 election approached, it became clear he would not seek re-election, and he was succeeded by Davies Gilbert.
5.2. Honours and Awards
Davy received numerous significant honors and awards throughout his career, reflecting his profound impact on science. He was awarded the Copley Medal in 1805, followed by multiple Bakerian Medals in 1806, 1807, 1808, 1809, 1810, 1811, and 1826. In 1807, he received the Galvanism Prize, a medal awarded by Napoleon Bonaparte for his electrochemical work. His invention of the Davy lamp earned him the Rumford Medal in 1816. In 1827, he was honored with the Royal Medal. His elevation to a Knight in 1812 and subsequently to a Baronet in 1818 further underscored his national recognition, making him the first British scientist to receive such a title.
5.3. European Tour with Faraday

In 1812, after being knighted and giving his farewell lecture at the Royal Institution, Davy married the wealthy widow Jane Apreece. (While generally faithful, their relationship was often stormy, and in later years, he traveled to continental Europe alone.) He then published Elements of Chemical Philosophy, part 1, volume 1, though other parts were never completed.
In October 1813, Davy and his wife, accompanied by Michael Faraday as his scientific assistant (who was also treated as a valet, as his wife's personal servant refused to travel to France, then an enemy nation), embarked on a tour of Europe. The primary purpose was for Davy to collect the second edition of the prix du Galvanisme medal, which Napoleon Bonaparte had awarded him for his electrochemical work. Faraday noted the unusual nature of the journey: "Tis indeed a strange venture at this time, to trust ourselves in a foreign and hostile country, where so little regard is had to protestations of honour, that the slightest suspicion would be sufficient to separate us for ever from England, and perhaps from life." Their party sailed from Plymouth to Morlaix by cartel, where they underwent searches.
Upon reaching Paris, Davy was a guest of honor at a meeting of the First Class of the Institut de FranceFrench and met with André-Marie Ampère and other French chemists. It was later reported that Davy's wife had thrown the Napoleon medal into the sea near her Cornish home, as it "raised bad memories." The Royal Society of Chemistry has since offered over 1.80 K GBP for its recovery. While in Paris, Davy attended lectures at the École Polytechnique, including those by Joseph Louis Gay-Lussac on a mysterious substance isolated by Bernard Courtois. Davy wrote a paper for the Royal Society on this substance, now known as iodine, leading to a dispute with Gay-Lussac over research priority. Davy's party did not meet Napoleon in person but visited Empress Joséphine de Beauharnais at the Château de Malmaison.
The party left Paris in December 1813, traveling south to Italy. In Florence, with Faraday's assistance, Davy conducted experiments using the Grand Duke of Tuscany's burning glass, successfully using concentrated sunlight to ignite a diamond, proving it is composed of pure carbon. They continued to Rome, where Davy experimented on iodine and chlorine and on the colors used in ancient paintings, marking the first chemical research on artists' pigments. He also visited Naples and Mount Vesuvius, collecting crystal samples. By June 1814, they were in Milan, where they met Alessandro Volta, then proceeded north to Geneva. They returned to Italy via Munich and Innsbruck. Plans to travel to Greece and Istanbul were abandoned after Napoleon's escape from Elba, prompting their return to England. After the Battle of Waterloo, Davy wrote to Lord Liverpool, urging severe treatment for the French, stating that France was "a conquered country" and that it was the allies' duty to impose "more restricted boundaries" and reclaim stolen wealth.
6. Personal Life
Davy's personal life was marked by a significant marriage, a complex mentorship that turned contentious, and a personality defined by both ambition and a love for nature.
6.1. Marriage and Family
In 1812, Humphry Davy married Jane Apreece, a wealthy widow. Their marriage was noted for being stormy at times, and in later years, Davy often traveled to continental Europe alone. The couple did not have any children.
6.2. Relationship with Michael Faraday

Davy's relationship with his laboratory assistant, Michael Faraday, was a complex mix of mentorship, collaboration, and later, bitter rivalry. Faraday was hired by Davy in 1813, partly due to injuries Davy sustained in a laboratory accident with nitrogen trichloride. Faraday went on to expand upon Davy's work and eventually became a more famous and influential scientist.
Davy is sometimes credited with having claimed Faraday as his "greatest discovery," though this statement is also reported as a "cruel joke" at Davy's expense, rather than a genuine acknowledgment. However, the relationship soured dramatically. In 1821, Davy accused Faraday of plagiarism regarding Faraday's independent discovery of electromagnetic rotation (the principle behind the first electric motor). According to Geoffrey Cantor's 1991 biography of Faraday, this "dubious accusation" was Davy's "final bid to dominate Faraday," stemming from resentment of his assistant's success and an attempt to "keep him down." This rivalry intensified when Davy tried to block Faraday's membership in the Royal Society in 1823, though he was ultimately unsuccessful, and Faraday became a member in 1824. This incident proved a turning point, causing Faraday to cease all research in classical electromagnetism until Davy's death in 1829.
6.3. Personality and Hobbies
Davy possessed a sanguine and somewhat irritable temperament, displaying characteristic enthusiasm and energy in all his pursuits. His mind was highly imaginative, as evidenced by his verses and prose. The poet Samuel Taylor Coleridge famously remarked that if Davy "had not been the first chemist, he would have been the first poet of his age," while Robert Southey added that "he had all the elements of a poet; he only wanted the art." Despite his sometimes ungainly exterior and peculiar manner, his exceptional gifts for exposition and illustration earned him extraordinary popularity as a lecturer. His experiments were ingenious and performed with remarkable speed, leading Coleridge to attend his lectures "to increase his stock of metaphors."
The dominating ambition of Davy's life was to achieve fame. While occasional petty jealousy surfaced, it did not diminish his concern for the "cause of humanity," a phrase he frequently used in connection with his invention of the miners' lamp. He was often careless about etiquette, and his frankness sometimes exposed him to annoyances that could have been avoided with more tact. Davy was also a deist, a belief he openly embraced, seeing God's design revealed through scientific investigation.
Beyond his scientific endeavors, Davy had a profound passion for fly-fishing, which earned him the informal title "the father of modern fly-fishing." His book Salmonia, published in 1828, is often considered "the fly-fisherman bible."
7. Later Life and Death
In his later years, Davy's health significantly declined. He suffered a stroke in 1826, from which he never fully recovered, leading to his resignation from the presidency of the Royal Society in 1827.
Davy spent the final months of his life writing Consolations in Travel, a posthumously published compendium of poetry, scientific thoughts, and philosophical reflections that became a staple in scientific and family libraries for decades. He spent the winter of 1828-1829 in Rome, hunting in the Roman Campagna on his fiftieth birthday. However, on February 20, 1829, he suffered another stroke. After several months spent attempting to recuperate, Sir Humphry Davy died in a room at L'Hotel de la Couronne, on Rue du Rhone, in Geneva, Switzerland, on May 29, 1829.

An appendix to his will included specific last wishes: no post-mortem examination, burial in Geneva where he died, and an interval between his death and burial to ensure he was not merely comatose. However, city ordinances did not permit such an interval, and his funeral took place on the following Monday, June 1, in the Plainpalais Cemetery (now Cimetière des Rois), located outside the city walls. His research and legacy were subsequently carried forward by his former assistant, Michael Faraday.
8. Legacy and Influence
Humphry Davy's lasting impact is evident in the fundamental changes he brought to chemistry, his practical inventions, and the numerous ways in which his contributions have been recognized scientifically, geographically, and culturally.
8.1. Scientific and Literary Recognition
Davy's legacy is honored in various ways. Shortly after his funeral, his wife arranged for a memorial tablet in Westminster Abbey at a cost of 142 GBP. In 1872, a statue of Davy was erected in front of the Market Building on Market Jew Street in Penzance, his hometown. A commemorative slate plaque at 4 Market Jew Street also marks his birthplace. Penzance further recognizes him with the Humphry Davy School and a pub named "The Sir Humphry Davy."
Beyond Penzance, the University of Plymouth has a science building named The Davy Building, and a road adjacent to the docks in Bristol is named Humphry Davy Way. In Sunderland, outside the Stadium of Light, a giant Davy Lamp stands as a tribute to local mining heritage and the lamp's importance. In Germany, there is a Humphry-Davy-Straße in Cuxhaven, and a satellite of the University of Sheffield in Wath upon Dearne was named Humphry Davy House until 2009. Geographical features also bear his name, including Davy Sound in Greenland, named by William Scoresby, and Mount Davy in New Zealand's Paparoa Range, named by Julius von Haast. In France, a former mining town, La Grand-Combe, has a commercial area called Parc régional d'activité économiques Humphry Davy.
Scientifically, the mineral davyne was named in his honor by W. Haidinger in 1827. Since 1877, the Royal Society of London has annually awarded the Davy Medal for "an outstandingly important recent discovery in any branch of chemistry." The Davy lunar crater, with a diameter of 21 mile (34 km), is also named after him.
Davy's passion for fly-fishing earned him the informal title "the father of modern fly-fishing," and his book Salmonia, published in 1828, is often considered "the fly-fisherman bible." His literary influence is also noted; the poet Samuel Taylor Coleridge famously said he "attended Davy's lectures to enlarge my stock of metaphors."
8.2. Criticism and Controversy
Despite his immense contributions, Davy's career was not without criticism and controversy. One significant point of contention revolved around the invention of the Davy lamp. While he was widely credited and awarded for it, there were debates over whether he had independently discovered its principles or if he had benefited from the work of others like William Reid Clanny and George Stephenson. Furthermore, some historical analyses suggest that while the lamp itself was effective in preventing explosions, its widespread adoption inadvertently led to an increase in mining fatalities. This was because the perceived safety of the lamp encouraged miners to work in previously abandoned, more dangerous, and gassier seams, thereby exposing them to other hazards.
Another notable controversy involved his relationship with his protégé, Michael Faraday. While Davy initially mentored Faraday and even reportedly claimed Faraday as his "greatest discovery," their relationship deteriorated. Davy later accused Faraday of plagiarism regarding Faraday's discovery of electromagnetic rotation in 1821. This accusation, seen by some as Davy's attempt to assert dominance and suppress his assistant's rising fame, led to significant tension. Davy actively opposed Faraday's election to the Royal Society in 1823, although Faraday was ultimately admitted. This incident caused Faraday to halt his research in classical electromagnetism until after Davy's death, highlighting the personal and professional strain between the two scientific giants.
Davy's character, described as sanguine and occasionally irritable, and his sometimes careless approach to etiquette, also drew criticism. He was known for his frankness, which, while a virtue, sometimes exposed him to unnecessary annoyances.
9. Publications
Humphry Davy was a prolific writer, publishing numerous books and scientific papers throughout his career. A comprehensive list of his articles can be found in June Z. Fullmer's work on his publications.
His major books include:
- Researches, Chemical and Philosophical; Chiefly Concerning Nitrous Oxide, or Dephlogisticated Nitrous Air, and Its Respiration (1800)
- Elements of Chemical Philosophy (1812)
- Elements of Agricultural Chemistry in a Course of Lectures (1813)
- The Papers of Sir H. Davy (1816), focusing on his safety lamp
- Discourses to the Royal Society (1827)
- Salmonia or Days of Fly Fishing (1828)
- Consolations in Travel or The Last Days of a Philosopher (1830), published posthumously
Davy also contributed articles on chemistry to Rees's Cyclopædia, though the specific topics are not known. His collected works were published posthumously by his brother, John Davy, in 1839-1840 under the title The Collected Works of Sir Humphry Davy.